Abstract
Background
Exposure to per- and polyfluoroalkyl substances (PFAS) has been linked with various cancers. Assessment of PFAS in drinking water and cancers can help inform biomonitoring and prevention efforts.
Objective
To screen for incident cancer (2016–2021) and assess associations with PFAS contamination in drinking water in the US.
Methods
We obtained county-level age-adjusted cancer incidence (2016–2021) from the Surveillance, Epidemiology, and End Results (SEER) Program. Data on PFAS levels in public drinking water systems were obtained from the Third (UCMR3; 2013–2015) and Fifth (UCMR5; 2023–2024) Unregulated Contaminant Monitoring Rule. UCMR3 measured PFOS, PFOA, PFNA, PFHxS, PFHpA, and PFBS. UCMR5 expanded measurements to include PFBA, PFHxA, PFPeA, and PFPeS. We created indicators of PFAS detection and, for UCMR5, concentrations above Maximum Contaminant Levels (MCLs). MCLs for PFOA and PFOS are 4 ng/L, and for PFNA and PFHxS are 10 ng/L. We used Poisson regression models to assess associations between PFAS detection or MCL violation and cancer incidence, adjusting for potential confounders. We estimated the number of attributable cancer cases.
Results
PFAS in drinking water was associated with increased cancer incidence in the digestive, endocrine, oral cavity/pharynx, and respiratory systems. Incidence rate ratios (IRRs) ranged from 1.02 to 1.33. The strongest association was observed between PFBS and oral cavity/pharynx cancers (IRR: 1.33 [1.04, 1.71]). Among males, PFAS was associated with cancers in the urinary, brain, leukemia, and soft tissues. Among females, PFAS was associated with cancers in the thyroid, oral cavity/pharynx, and soft tissue. PFAS in drinking water is estimated to contribute to 4626 [95% CI: 1,377, 8046] incident cancer cases per year based on UCMR3 data and 6864 [95% CI: 991, 12,804] based on UCMR5.
Impact statement
The ecological study examined the associations between PFAS in drinking water measured in two waves (2013–2015 and 2023–2024) and cancer incidence between 2016 and 2021. We found that PFAS in drinking water was associated with cancers in the organ system including the oral cavity/pharynx, lung, digestive system, brain, urinary system, soft tissue, and thyroid. Some cancers have not been widely studied for their associations with PFAS. We also observed sex differences in the associations between PFAS and cancer risks. This is the first ecological study that examined PFAS exposure in drinking water and various cancer risks.
Similar content being viewed by others
Introduction
Per- and polyfluoroalkyl substances (PFAS) are synthetic chemicals that have been widely used in consumer products and have accumulated in the environment since the 1940s due to their resistance to degradation [1]. Drinking water is a major source of exposure for the general population [2]. One recent study suggests that one or more types of PFAS were detected in at least 45% of drinking water across the US near urban areas and potential PFAS sources [3]. Smalling et al. found that 40% of the public water had PFAS detected compared to 20% of the private-well samples although the concentrations of individual PFAS were not different between public and private-well water samples. On April 10, 2024, the US Environmental Protection Agency (EPA) announced the final National Primary Drinking Water Regulation which sets the Maximum Contaminant levels (MCL), a legally enforceable level of contaminants in drinking water, for 6 PFAS [4]. The unprecedented rulings regulating PFAS in drinking water demonstrate the importance of assessing the health risks of PFAS from drinking water.
It is estimated that there were 1.9 million new cancer cases and more than 600,000 deaths from cancers in the US in 2023 [5]. Endocrine-disrupting chemicals like PFAS have been proposed as environmental risk factors for cancers through multiple mechanisms, such as alterations in male and female reproduction, epigenetic changes, and changes in neuroendocrinology, behavior, and metabolism which can lead to the development of cancers [6, 7].
PFAS have been linked to various health outcomes including cancers [8] and the International Agency for Research on Cancer (IARC) has listed PFOA as carcinogenic to humans (Group 1) and PFOS as possibly carcinogenic to humans (Group 2B) [9]. A systematic review and meta-analysis identified associations between PFAS and kidney and testicular cancers [10]. In addition, the National Academies of Sciences, Engineering, and Medicine (NASEM) conducted reviews of evidence for health risks of PFAS and concluded sufficient causal evidence existed for the associations between PFAS and kidney cancer, suggestive evidence for associations between PFAS and breast and testicular cancer [11].
Several mechanisms were identified including endocrine disruption, lipid metabolism, epigenetic change, oxidative stress, chronic inflammation, and immunosuppression [12]. For example, PFAS can bind to nuclear receptors such as peroxisome proliferator-activated receptors which regulate the lipid metabolism although evidence is less clear for the direct binding to estrogen and androgen receptors [6]. Disruption in hormone homeostasis is implicated in the mechanisms causing liver, thyroid, prostate, and breast cancers [12]. PFAS have also been linked to both hyper- and hypomethylation of DNA and functional analysis of the differentially methylated probes or regions showed the involvement of cancer and reproductive disease [12, 13]. Therefore, there is strong biological plausibility to link PFAS with cancers.
In 2022, approximately half of the new cancer cases were cancers of the prostate, breast, lung, colon, and rectum [14] but existing epidemiological studies were limited to the associations between PFAS and these cancers. In addition, fewer studies focused on the source-specific effect of PFAS, and most of these studies were based in European countries [15] except for studies from the C8 Health Project [16, 17], one study examining the correlation between PFAS in water and thyroid cancer in the US [18] and the associations between PFOA in water and cancer risks in the Mid-Ohio River Valley [16]. Identifying the contribution to cancer risk from PFAS in drinking water would help prioritize and target drinking water quality to reduce risk. Thus, there is an urgent need for more health assessment of cancer risks from PFAS, especially from drinking water.
We hypothesized that there are cancers caused by PFAS in drinking water that have not been identified previously due to the limited sample size of cancer cases and lack of PFAS quality data. We aimed to screen for associations between PFAS in drinking water and county-level cancer incidence in an ecological study that would help direct future epidemiological and experimental research on PFAS and cancer. We used data from the Third Unregulated Contaminant Monitoring Rule (UCMR3) [19], and the Fifth Unregulated Contaminant Monitoring Rule (UCMR5) [20] in public drinking water systems (PWS), and cancer incidence data from the Surveillance, Epidemiology, and End Results (SEER) Program between 2016 and 2021. To better understand the cancer burden due to PFAS and provide a policy-relevant perspective, we additionally estimated the number of cancer incidence cases attributable to PFAS in drinking water.
Method
Cancer incidence data
We obtained cancer incidence data from the U.S. National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) program [21]. The geographic coverage of the SEER database, which includes 22 cancer registries, is approximately half of the US population. The geographic area coverage included Connecticut, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, Alaska Natives, Georgia, California, Kentucky, Louisiana, New Jersey, Idaho, New York, and Texas. The data are publicly available and can be obtained through the SEER*Stat program [21].
County-level age-adjusted incidence rates between 2016 and 2021 (patients with diagnosis of cancers between 2016 and 2021) were calculated using the SEER*Stat program using “Incidence – SEER Research Limited-Field Data, 22 Registries, Nov 2023 (2000-2021)” database [21]. The crude rate was age-adjusted according to the 2000 US population. We obtained yearly, age-adjusted cancer incidence rates per 100,000 for each county and each cancer site. The cancer site was based on the ICD-O-3/WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (2008), grouped as follows: oral cavity and pharynx, digestive system, respiratory system, bones and joints, soft tissue including heart, skin excluding basal and squamous (excluding non-melanoma cases), breast, female genital system, male genital system, urinary system, brain and other nervous system, endocrine system, lymphoma, myeloma, leukemia. We excluded cancers in the eye and orbit, mesothelioma, and Kaposi sarcoma due to the limited number of cases. We assessed the associations between PFAS and cancers in the male/female genital system in stratified analyses (excluded from the main analysis since sex-specific incidence rate is a more appropriate outcome).
PFAS in drinking water
We obtained two different sets of PFAS data: (1) US EPA’s Third Unregulated Contaminant Monitoring Rule (UCMR3) data (2013 to 2015) [19] and (2) US EPA’s Fifth Unregulated Contaminant Monitoring Rule (UCMR5) data (2023 to March 2024) [20].
UCMR3 was conducted by the US EPA between January 2013 and December 2015 under the Safe Drinking Water Act and monitored unregulated contaminants in PWS including 6 PFAS: perfluorooctanesulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA), perfluorohexanesulfonic acid (PFHxS), perfluoroheptanoic acid (PFHpA), and perfluorobutanesulfonic acid (PFBS). UCMR3 tested PFAS in all PWSs serving more than 10,000 people and 800 representative PWSs serving 10,000 or fewer people across all states in the US. The minimum reporting levels (MRLs) for PFOS, PFOA, PFNA, PFHxS, PFHpA, and PFBS were 0.04, 0.02, 0.02, 0.03, 0.01, and 0.09 µg/L, respectively. The detection rates for PFOS, PFOA, PFNA, PFHxS, PFHpA, and PFBS across all samples were 0.79%, 1.03%, 0.05%, 0.56%, 0.64%, and 0.05%.
UCMR5 started in 2023 and the most recent release of data was up to March 2024 [20]. 29 PFAS were tested by UCMR5 and MRL for these PFAS ranges from 2 to 20 ng/L. We included PFAS that were commonly present in PWS including 6 PFAS mentioned above plus perfluorobutanoic acid (PFBA), perfluorohexanoic acid (PFHxA), perfluoropentanoic acid (PFPeA) and perfluoropentane sulfonic acid (PFPeS) because these additional PFAS were detected in the water systems. We hypothesize that UCMR5 was a valid measure of exposure because of the lower detection of limit for each PFAS, potentially correcting the exposure misclassification in UCMR3.
We downloaded the geographic boundaries of PWSs for the United States [22] and linked each of the PFAS data to the PWS boundaries. Briefly, SimpleLab used three approaches to identify boundaries of water systems: (1) use water service boundaries provided by each state, (2) matching algorithm to match water systems to the boundary of a town or city, (3) use a statistical model to estimate the reasonable radium of a water service boundary. Therefore, misclassification of water boundaries is likely for the second and third approaches. We calculated the average PFAS concentrations detected for each PWS and each PFAS chemical by different data sources.
Since no detection of PFAS could mean either a water system had no PFAS or had PFAS concentrations below the detection limit and we had to average across values across multiple samples collected for the same water system and for each county, using the continuously measured concentrations for each PFAS would not be meaningful. In addition, detection limits for UCMR3 are much higher and a wide range of values of concentrations was possible. Thus, imputation is not appropriate.
For UCMR3 data, we created a binary variable indicating the detection of PFAS in the drinking water for each county.
We created another binary variable indicating the violation of Maximum Contaminant Levels (MCLs), which are legally enforceable levels of contaminants in drinking water. MCLs for PFOA and PFOS are 4 ng/L and MCLs for PFNA and PFHxS are 10 ng/L. Since there were no individual MCLs for other PFAS, we only created detected/not detected variables for other PFAS. We only created this variable for UCMR5 not for UCMR3 because the MRL in UCMR3 was much higher than MCL.
We additionally created a binary variable indicating at least 1 PFAS was detected in drinking water separately for UCMR3 and UCMR5, and another binary variable indicating each of the PFAS was detected/above MCL in both UCMR3 and UCMR5.
Covariates
We obtained 5-year estimates of American Community Survey data from 2013 to 2017. We calculated the following county-level socioeconomic status (SES) variables: percent of people of color (including any race with Hispanic ethnicity)/non-White, percent of the population (25 years and over) with education below high school, percent of the population whose income was below the federal poverty line, and percent of the population in the workforce that was unemployed.
We obtained additional potential confounders including air pollution, obesity prevalence, smoking rate, and urbanicity. We included air pollution as a proxy for co-occurring environmental pollutants with PFAS contamination in drinking water since areas with high industrial activities would have potentially high air pollution levels due to traffic [23,24,25,26]. The data source for each covariate is described below.
We downloaded 2018 annual average fine particulate matter (PM2.5; unit: μg/m3, resolution: 0.01° × 0.01°) data [27, 28] and calculated county-level average PM2.5. PM2.5 was estimated using Aerosol Optical Depth (AOD) retrievals from the NASA MODIS, MISR, and SeaWIFS instruments with the GEOS-Chem chemical transport model, and subsequently calibrated to regional ground-based observations of both total and compositional mass using Geographically Weighted Regression (GWR).
Urbanicity was classified based on the 2013 NCHS Urban-Rural Classification Scheme for Counties which classified counties into the large central metro, large fringe metro, medium metro, micropolitan, and noncore [29]. The metropolitan area includes a large metro (with a metropolitan statistical area (MSA) population of 1 million or more; divided into large central metro, large fringe metro); medium metro (with MSA population between 250,000 and 999,999), small metro (with MSA population less than 250,000). Non-metropolitan areas include micropolitan (with urban cluster population between 10,000 and 49,999) and noncore.
Obesity prevalence and prevalence of smokers in 2018 (% of the population that was obese) were obtained from the CDC’s Behavioral Risk Factor Surveillance System (BRFSS) [30].
Statistical methods
Association study
We assessed the associations between individual PFAS and county-level cancer incidence rates using negative binomial regression adjusting for county-level SES variables, urbanicity, smoking rate, obesity, and air pollution. We chose the negative binomial model due to overdispersion which violated the assumption of the Poisson model. We calculated incidence rate ratios (IRR) and 95% confidence interval. We conducted the following analysis:
-
1.
Detection of PFAS (2013–2015) and county-level annual average cancer incidence rate per 100,000 (2016–2021)
-
2.
Detection or Violations of MCLs of PFAS (2023–2024) and county-level annual average cancer incidence rate per 100,000 (2016–2021)
Where Y was the incidence rate of cancer,
PFAS was the detection of or MCL violation of PFAS in drinking water,
C was the potential confounders described above,
Population is the population size for each county
We adjusted for multiple tests using the false discovery rate (FDR) method across major cancer systems for each PFAS. We used a significance threshold of 0.05 for FDR-adjusted p-values. However, given the exploratory nature of the study, we presented all findings at the crude p-value of 0.05.
To reduce the number of false positive associations, we only evaluated and presented the associations between PFAS and subtypes of cancers within each major organ system when PFAS was associated with the cancers of that major organ system at a crude p-value of 0.05. However, full summary statistics were provided for reference across all-cancer subtypes. We did not further conduct multiple testing corrections among subtypes within a major organ system because some of the subtypes are the primary driver of the overall cancer incidence in that major organ system (i.e. lung cancer in respiratory) and some of the categories contain overlapping organs (i.e. cancer in liver and intrahepatic bile duct contains both liver and intrahepatic bile duct cancers).
To account for potential outcome clustering, we additionally conducted a sensitivity analysis using a generalized mixed-effect model by including a random intercept at the state level.
Where i was each county and j was each state.
We conducted two additional sensitivity analyses (1) assessing the association between detection/MCL violation of at least 1 PFAS in drinking water and cancer incidence separately for UCMR3 and UCMR5 and (2) detection/MCL violation of PFAS in both UCMR3 and UCMR5 and cancer incidence. By collapsing different PFAS exposures, we would greatly reduce the exposure misclassification and when we assessed the continuous detection of PFAS in water systems, we can both limit the exposure misclassification and identify long-term PFAS contamination.
Additional sensitivity analyses were done to assess the influence of covariates in the model including (1) removing air pollution from the model, (2) removing obesity from the model, (3) adding the number of potential PFAS-polluting industrial sites in each county-based data obtained from the PFAS Analytic Tools [31]. Briefly, the EPA developed a dataset from various sources to show which industries may handle and/or release PFAS in their facilities, but the data does not mean that the included facilities were actively manufacturing, processing, using, or releasing PFAS.
Incident cancer case attributable to PFAS
We calculated attributable cancer cases if the associations were statistically significant based on a crude p-value cutoff of 0.05. We first calculated population attributable fraction (PAF) based on IRR:
Where:
P is the proportion of the population exposed to PFAS.
IRR is the incidence rate ratio for the association between PFAS and cancer incidence.
The proportion of the population exposed to PFAS was calculated by the following:
Where:
N is the total number of counties.
Pop is the population for each county.
Detection or MCL violation of PFAS is the binary variable indicating the county had PFAS detected in their drinking water or the county’s PFAS concentration was above the MCL (1 is yes and 0 is no).
Lastly, we calculated the number of incident cancer cases attributable to PFAS:
Where:
The total US population in 2023 was n = 339,996,563.
Incidence Rate per 100,000 was calculated as the average incidence rate for all counties.
We estimated 95% CI using the 95% CI of the effect estimates from the association study.
We then summed all-cancer cases attributable to each PFAS.
Where Attributable CasesPFAS is the estimated attributable cancer cases by each PFAS.
Stratification by sex
Lastly, we obtained sex-specific age-adjusted yearly cancer incidence between 2016 and 2021. We then assessed the association between PFAS from UCMR3 and UCMR5 and cancer incidence rate by sex using a negative binomial model.
Results
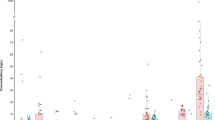
Figure 1 shows an example of the distribution of PFOA in UCMR3 and UCMR5 and respiratory and endocrine cancer incidence. Distribution of other cancer incidence can be found in Supplemental Figs. 1–14. Table 1 shows that we included data from 1080 counties, encompassing a population of approximately 156.1 million (roughly half of the US population). PFAS data were available for 686 counties based on UCMR3 and 663 or 665 counties based on UCMR5.
A Detection of PFOA in drinking water based on UCMR3, B Maximum Contaminant Level (MCL) violation of PFOA based on UCMR5, C Annual average cancer incidence rate per 100,000 (between 2016 and 2021) for cancers in the respiratory system, and D Annual average cancer incidence rate per 100,000 (between 2016 and 2021) for cancers in the endocrine system.
At the water system level, when we looked at the six overlapping PFAS (PFOA, PFOS, PFHpA, PFHxS, PFNA, and PFBS) and their detection based on UCMR3 and UCMR5 across 2351 water systems, the majority of the PFAS were not detected in both UCMR3 and UCMR5 (87%) and roughly 1% had detection both in UCMR3 and 5. 12% were only detected by UCMR5 and about 1% only by UCMR3. At the water system levels, data from UCMR3 and UCMR5 were fairly correlated (Pearson’s correlation coefficient: 0.16, p-value < 2.2e-16).
At the county level, the most detected PFAS in drinking water based on UCMR3 were PFOA and PFOS, followed by PFHpA, PFHxS, PFNA, and PFBS (7.4%, 5.5%, 5.5%, 4.4%, 1%, and <1%, respectively). Based on UCMR5, violations of the MCL for PFOA, PFOS, and PFNA were 7.7%, 7.7%, and 2.1%, respectively, while detections of PFBA, PFPeA, PFHxA, PFBS, and PFHpA were 33%, 29%, 28%, 33%, and 12%.
Associations between PFAS and cancers
Figures 2 and 3 show the associations between PFAS detection in drinking water based on UCMR3 (exposure period: 2013–2015) and UCMR5 (exposure period: 2023–2024) and cancer incidence rates (2016–2021). Overall, we found four types of cancers were positively associated with the detection of or an MCL violation of at least one PFAS based on UCMR3 or UCMR5 data including the digestive system, endocrine system, oral cavity and pharynx, and respiratory system (IRR ranges: 1.02–1.33; see Supplemental Table 1–3).
The largest effect was observed between the detection of PFBS and cancers in the oral cavity and pharynx (IRR: 1.33 [1.04, 1.71], p-value: 0.02, FDR-adjusted p-value: 0.33). This was followed by the detection of PFNA in UCMR3 and PFHpA in UCMR5, which were associated with cancers in the endocrine system, mainly thyroid cancers (IRRs: 1.28 [1.04, 1.57], p-value: 0.01, FDR-adjusted p-value: 0.18 and 1.10 [1.01, 1.19], p-value: 0.02, FDR-adjusted p-value: 0.33).
Detection of PFBA and MCL violations of PFHxS in UCMR5 were positively associated with digestive system cancers. Specifically, these cancers were located in the large intestine (IRR: 1.20 [1.07, 1.34]) and liver (IRR: 1.10 [1.03, 1.18]) for PFBA. For PFHxS, the cancers were located in the esophagus (IRR: 1.37 [1.09, 1.72]), colon and rectum (IRR: 1.12 [1.03, 1.21]), rectosigmoid junction (IRR: 1.36 [1.00, 1.83]), and gallbladder (IRR: 1.60 [1.06, 2.41]; see Supplemental Tables 4–6).
Additionally, the detection of PFOA in UCMR3 and PFBA in UCMR5 was associated with respiratory system cancers, mainly lung cancers (IRRs: 1.08 [1.02, 1.14] and 1.03 [1.01, 1.06]).
Lastly, the detection of PFBA was associated with a reduced risk of skin cancer and leukemia (IRRs: 0.89 [0.84, 0.93], p-value: <0.01, FDR-adjusted p-value: <0.01 and 0.96 [0.91, 1.00], p-value: 0.05, FDR-adjusted p-value: 0.54). Full summary statistics are available in Supplemental Table 1–6.
Cancer cases attributable to PFAS
As shown in Table 2, we estimated that the detection of PFBS, PFNA, and PFOA would contribute to 4626 [95% CI: 1377, 8046] incident cancer cases each year based on UCMR3 data while based on UCMR5 data, detection of PFBA and PFHpA and MCL violation of PFHxS would contribute to 6864 [95% CI: 991, 12,804] incident cancer cases each year.
Mixed-effect model
When we conducted a generalized linear mixed model with a random intercept at the state level (as a sensitivity analysis), the detection of PFOA in UCMR3 was associated with an increased risk of lung cancers (IRR: 1.06 [1.01, 1.11]). In UCMR5, we observed associations between PFHxS and an increased risk of digestive system cancers (IRR: 1.12 [1.05, 1.19]), PFHpA and endocrine system cancers (IRR: 1.10 [1.02, 1.19]), and both PFPeA and PFHpA with respiratory system cancers (IRRs: 1.03 [1.00, 1.06] and 1.04 [1.00, 1.08]). Overall, the results were fairly similar to our main analysis. See Supplemental Tables 7–9 for all summary statistics.
Sensitivity analysis
As shown in Supplemental Tables 10 and 11, when we collapsed detection/MCL violation of PFAS in drinking water into one variable (at least 1 PFAS detected/had MCL violation), detection of at least 1 PFAS in drinking water was associated with cancers in the respiratory system based on both UCMR3 and 5. However, none of the associations were significant at an FDR-adjusted p-value threshold of 0.05. As shown in Supplemental Table 12, only detection/MCL violation of PFOA in both UCMR3 and 5 was associated with increased risk of cancers in all-cancer incidence and cancer incidence in the endocrine system and reduced risk of skin cancer.
When we removed air pollution from the model, we found that the detection of PFOA in UCMR3 was positively associated with cancers in the respiratory system, and the detection of PFBA was negatively associated with skin cancer at FDR-adjusted p-value of 0.05 (Supplemental Table 13). When we removed the prevalence of obesity, we found that MCL violation of PFHxS in UCMR5 was positively associated with cancers in the digestive system, detection of PFBA in UCMR5 was negatively associated with cancers of the skin and endocrine system, detection of PFOA in UCMR3 was positively associated with cancers in the respiratory system at FDR-adjusted p-value of 0.05 (Supplemental Table 14).
When we additionally controlled for the number of potential PFAS-polluting facilities in the county, MCL violation of PFHxS in UCMR5 was positively associated with the digestive system, detection of PFBA in UCMR5 was negatively associated with skin cancer, and detection of PFOA in UCMR3 was positively associated with respiratory cancer at FDR-adjusted p-value of 0.05 (Supplemental Table 15).
Sex-stratified analysis
Among males, we found four types of cancers were positively associated with the detection or an MCL violation of PFAS based on UCMR3 or UCMR5 data including cancers in the urinary system, brain and other nervous system, leukemia, and soft tissues. Among females, we found three types of cancers were positively associated with the detection or an MCL violation of PFAS based on UCMR3 or UCMR5 data including cancers in the endocrine system, oral cavity and pharynx, and soft tissue.
Based on UCMR3, we found significant associations between the detection of PFHpA and increased risk of brain cancers (IRR: 1.22 [1.02, 1.47]), the detections of PFHxS and PFOS and increased risk of cancers in the urinary system (IRRs: 1.11 [1.00, 1.22], 1.10 [1.01, 1.20]), the detection of PFOS and reduced risk of myeloma (IRR: 0.8 [0.70, 0.99]) among males. In females, we found associations between the detection of PFOA and increased risk of cancers in the oral cavity and pharynx as well as reduced risk of skin cancer (IRR: 1.20 [1.03, 1.39], 0.85 [0.73, 0.98]) (see Supplemental Table 16).
Based on UCMR5, MCL violations of PFOS, and PFOA and increased risk of cancers in soft tissue in both male and female groups (male: 1.66 [1.33, 2.06], 1.75 [1.42, 2.16]; female: 1.56 [1.24, 1.97], 1.32 [1.05, 1.67]).
Among males, we found associations between MCL violation of PFOA and detection of PFHpA and increased risk of brain cancers (IRRs: 1.20 [1.02, 1.40] and 1.15 [1.00, 1.32]), MCL violations of PFHxS and PFOS and detection of PFPeA and increased risk of leukemia (1.86 [1.50, 2.29], 1.19 [1.05, 1.34], and 1.09 [1.01, 1.17]), detections of PFBS, PFPeA, PFHxA, and MCL violation of PFHxS and increased risk of cancers in soft tissues (IRRs: 1.19 [1.02, 1.37], 1.24 [1.07, 1.43], 1.28 [1.10, 1.48], 4.13 [2.93, 5.82]), and MCL violation of PFHxS and increased risk of cancers in urinary system (IRR: 1.28 [1.12, 1.47]). We also found that MCL violation of PFHxS was associated with a reduced risk of lymphoma and cancers in the male genital system among males (IRRs: 0.75 [0.63, 0.91] and 0.86 [0.77, 0.96]).
Among females, we found detections of PFHpA, PFHxA, and PFPeA were associated with endocrine cancers (IRRs: 1.15 [1.03, 1.29], 1.11 [1.01, 1.21] and 1.09 [1.00, 1.19]) and MCL violation of PFOS and cancers in the oral cavity and pharynx (IRR: 1.25 [1.07, 1.46]) (see Supplemental Tables 17 and 18).
Discussion
Our study was the first ecological study to assess cancer-wide associations with PFAS exposure in drinking water using cancer incidence data from SERR between 2016 and 2021 and PFAS data in drinking water from UCMR3 (2013 to 2015) and UCMR5 (2023 to 2024). We found significant associations between PFAS in drinking water including PFOS, PFBS, PFHxS, PFOA, PFNA, PFBA, and PFHpA, and increased incidence rates of cancers in oral and pharynx, digestive, respiratory, and endocrine systems. In the sex-stratified analysis, we found that in males PFAS were associated with cancers in the urinary system, brain and other nervous system, leukemia, and soft tissues, and in females, PFAS were associated with the endocrine system, oral cavity and pharynx, and soft tissue. Some of the cancers identified in our study have not been studied for their associations with PFAS. In addition, based on UCMR3 and 5, we estimated that 4626 [95% CI: 1377, 8046] and 6864 [95% CI: 991, 12,804] cases were attributed to PFAS in drinking water.
Drinking water is a significant route of PFAS exposure for the general population [2]. Recent studies further corroborate that PFAS levels in the blood are driven by drinking water exposure using matched tap water and plasma samples [32, 33]. Existing literature provides robust evidence linking PFAS to kidney cancer and suggestive evidence for breast and testicular cancers [11, 34]. However, most studies have focused on PFOS and PFOA, with limited research on other PFAS and their associations with cancers, and there is limited research assessing source-specific health effects of PFAS, especially from drinking water [15,16,17, 35, 36].
Digestive system
Our study also found that detection of PFBA and MCL violation of PFHxS in drinking water were associated with increased risks of cancers in the digestive system, including the esophagus (esophageal cancer), colon and rectum (colorectal cancer), rectosigmoid junction, liver (liver cancer/hepatocellular carcinoma), and gallbladder cancers. Although previous studies did not identify associations between PFHxS and PFBA with esophageal cancer, a recent National Health and Nutrition Examination Survey (NHANES) study using data from 2003 to 2018 suggested increased risks of esophageal cancer with PFOA and PFOS exposure [37]. Since PFAS-contaminated drinking water would directly expose tissues in the esophagus, the associations with PFBA and PFHxS are thus plausible and deserve further investigation.
For liver cancer, consistent evidence from both rodent and human observational studies suggests that PFAS can cause liver injury [38], leading to a higher risk of liver cancer [39]. In a mouse study, exposure to PFBA showed increased liver weight and changes in gene expression in the liver [40]. One previous study also suggested that PFBA was preferentially accumulated in liver and lung cancers [41]. Several studies have also demonstrated that PFAS can disrupt lipid metabolism, which plays a crucial role in the development and progression of liver cancer [42,43,44,45]. Our study adds evidence that the detection of PFBA was associated with an increased risk of liver cancer.
We found that violation of MCL of PFHxS was associated with colorectal cancers specifically in males. NASEM identified an association between PFAS exposure and ulcerative colitis, a chronic inflammatory bowel disease (IBD) [11]. Patients with IBD are at increased risk of colorectal cancer and intestinal lymphoma [46]. Experimental studies in mice have shown that ingestion of PFOA-treated water may induce alterations in epigenetic and tight junction genes in the small intestine and colon, as well as changes in the gut microbiome [47,48,49]. The mucosal barrier and gut microbiota collectively maintain the homeostasis of the gastrointestinal (GI) tract, and damage to the intestinal barrier can lead to colorectal cancer [50, 51]. However, human observational studies of PFAS and colorectal cancer found mixed results. One study in the mid-Ohio Valley showed an inverse association between PFOS and colorectal cancer [52]. In a Swedish cohort, residents living in highly contaminated areas had elevated cancer risk in the rectum [15]. More studies are needed to evaluate colorectal cancer risk due to PFAS in both human and animal studies.
Respiratory system
We found that detections of PFOA and PFBA were associated with an increased risk of lung cancer. In sex-specific analysis, we found several PFAS associated with cancers in the lung including pleura and trachea in both males and females, although these PFAS were not associated with cancer in the entire respiratory system. Moon and Mun (2024) found that PFOS and PFNA were associated with an increased risk of lung cancer (ORs: 2.62 [1.24–5.83] and 2.38 [1.00–5.52]). In the Swedish cohort, living in areas contaminated with PFAS was associated with lung cancer risk among men but not among women [15]. In a study among lung cancer patients, PFAS including PFOA in blood was associated with multiple clinical indicators related to immune and liver functions [53]. Exposure to PFAS, through both inhaled and non-inhaled pathways, can target the lungs and modify lung functions and inflammatory responses. A study exposing human bronchial epithelial cells to PFOA and PFOS showed activated inflammasome, altered membrane properties of cells, and effects on inflammation- and immune-related genes [54]. In addition, PFBA was detected at a higher concentration in liver and lung tissues in humans [41]. Therefore, it is plausible that PFOA and PFBA cause lung cancer development.
Endocrine system
We observed that detections of PFNA and PFHpA were associated with an increased risk of thyroid cancer and the associations were primarily in females. PFAS are also considered endocrine-disrupting chemicals (EDCs) and can therefore increase the cancer risk of hormone-sensitive organs, including the thyroid [55]. PFAS may disrupt the thyroid hormone system based on in vitro and animal studies, leading to impaired thyroid function and an increased risk of thyroid cancer [56, 57]. Other studies also found that exposure to PFOA and PFOS was associated with disrupted thyroid hormones and alterations in thyroid gene expression [58, 59]. However, epidemiological evidence for the associations between PFAS and thyroid cancer is inconsistent. One case-control study in China found that PFNA, PFDA, and PFUnDA were associated with a lower risk of thyroid cancer [60]. Similar inverse associations were found in several studies, including a Finnish study [61] and another study in China [62]. Conversely, a recent study in the US found positive associations between PFOS and thyroid cancer [63]. Our study further suggests that there is a potential interaction between female sex and PFAS associations with thyroid cancer development. Since thyroid cancer is more common in females than males, it is plausible that sex hormone plays a role in the development of thyroid cancer and thus can interact with PFAS leading to greater thyroid cancer risk [64].
Urinary system
In males, we also found that PFOS and PFHxS were associated with cancers in the urinary system including kidney cancer and bladder cancer. A recent systematic review identified an overall association between PFOA and PFOS and kidney cancer [10]. Although we did not observe associations between kidney cancer and PFOA in our main analysis, in sex-stratified analysis the IRR [95% CI] between PFOA and kidney cancer was 1.09 [0.99, 1.19], p-value: 0.08 in UCMR3, comparable to the C8 study associations (HR: 1.10; 95% CI: 0.98, 1.24) [16, 17]. Studies included in the systematic review also found sex-specific associations between PFAS and kidney cancers in males only [10].
Previous evidence suggests that the kidney is a major pathway for PFAS elimination from the human body and PFAS can cause damage to kidney function over time leading to possible kidney cancer [65]. Future studies should consider evaluating PFAS other than PFOA and PFOS to assess nephrotoxicity and sex-specific effects of these PFAS on kidneys.
Hematologic system (blood and lymphatic)
We found inverse associations between the detection of PFBA and leukemia, but sex-specific effect estimates suggest that MCL violations of PFHxS and PFOS and the detection of PFPeA were positively associated with leukemia in males. Two epidemiological studies found PFAS were associated with leukemia in childhood or adulthood [35, 66] and an Italian study also found higher leukemia in males only [35].
Nervous system
In the male group, we also found several PFAS including detection of PFHpA and violation of MCL of PFOA were associated with brain cancer. Growing evidence suggests that PFAS have neurotoxicity. Previous experimental studies show that PFAS may cross the blood-brain barrier to cause damage to brain cells and disrupt neurotransmission [67]. PFAS was found in 96% of the samples of brain tissues from brain cancer patients [68]. Moon and Sun (2024) also found that PFHxS was associated with increased brain cancer in NHANES.
Skin and soft tissue
We found inverse associations between the detection of PFBA and skin cancer, which are not plausible. Dermal exposure to consumer products contains high concentrations of PFAS (eg. 22–10,500 ng/g product weight in cosmetics found in North America) [69]. PFAS has been detected in almost all cosmetics and personal care products [70]. In addition, the SEER database did not cover some states with higher PFAS contamination. More studies are needed to evaluate the effects of PFAS on skin cancer.
We also found that PFAS was associated with cancer in soft tissues. Few previous studies have identified a link between PFAS and soft tissue [71,72,73]. Therefore, further study is needed on the effects of PAS on specific soft tissue.
Head and neck
Our study identified significant associations between the detection of PFBS in drinking water and an increased risk of oral and pharynx cancer. Among females, detection of PFOA was associated with oral and pharyngeal cancers. EPA’s toxicity assessment of PFBS through evidence synthesis of existing experimental and epidemiological studies and calculation of toxicity levels suggested that PFBS is less toxic than PFOA and PFOS and in humans, only asthma and serum cholesterol levels were associated with PFBS [74]. Previous studies often reported null associations between PFBS as well as other PFAS and oral/pharynx cancers [75], perhaps due to small sample sizes since oral and pharyngeal cancers are relatively rare, accounting for about 3% of all-cancer cases [76]. Diet and drinking water are the primary routes of PFAS exposure [77], leading to direct contact with PFAS in the mouth and pharynx. Mechanistic studies on PFAS and oral/pharynx cancer are limited. However, PFAS may induce cancer through mechanisms such as oxidative stress and DNA damage in squamous cells [6] or by increasing permeability and damaging the epithelial cells lining the oral cavity and pharynx, creating an environment for cancer development [78]. More research is needed to confirm our findings, as no previous studies have specifically examined PFAS effects on oral or pharyngeal cells.
Eye and orbit
Although we were not able to examine the associations between PFAS and cancers in the eye and orbit, there has been a recent study on PFAS measured in metabolomics and retinoblastoma in childhood [79] and they found that PFOS was associated with retinoblastoma and PFOA was associated with retinoblastoma only in children of US-born mothers. Therefore, future studies can further assess the associations between PFAS and retinoblastoma in adulthood to strengthen the findings.
Inconsistency of results between UCMR3 and UCMR5
We observed differences in the results when using UCMR3 and UCMR5. First, since we observed that more water systems had detection of PFAS in UCMR5, it is unlikely that those water systems recently increased in PFAS contamination due to increased attention to PFAS contamination and the recent announcement of EPA’s rule on MCL of six PFAS [4]. The more likely explanation is the improved quantification which resulted in much lower detection limits in UCMR5 than UCMR3 and thus UCMR5 might be a more accurate measure of PFAS levels in drinking water. However, it is also possible that exposure in UCMR5 is misclassified which leads to differences in the results (described below in the limitation).
Sex-specific PFAS and cancer associations
We found that different cancers were associated with PFAS by sex. PFAS have been previously found to have sex-specific effects on various health outcomes including cardiovascular diseases, cholesterol metabolism, liver disease, and neurodevelopment [80,81,82]. In PFAS and cancer research, in an analysis in NHANES, PFAS were only associated with melanoma among women but not among men [83]. In addition, in another prospective study of cancer, they found that PFOA was associated with renal cell carcinoma among women while PFHxS was associated with leukemia among men [84]. There have been studies suggesting that half-lives of PFAS were different in males and females which were not explained by reproductive factors [85, 86]. In addition, there have been studies suggesting sex-specific effects of PFAS on biological mechanisms such as cholesterol metabolism, liver injury, and inflammatory biomarkers [80, 87, 88]. Therefore, future studies should focus on the sex-specific effects of PFAS on cancers.
Strength and limitations
By focusing on this source-specific PFAS exposure, we provide actionable targets for interventions, such as water quality, to policymakers. We categorized PFAS levels according to the new EPA regulatory standards (final MCLs announced in 2024). By using these new MCLs as cutoffs, our analysis gains increased policy relevance. We controlled for numerous area-level confounders and explored various modeling approaches. In our main analysis, we controlled for age and conducted sex-stratified analyses to account for confounding by sex.
Our study had several limitations. It was an ecological study analyzed at the county level, and thus we were not able to control individual-level confounders except for age and sex. We were also unable to control for potential confounders specific to each cancer type.
Although SEER covers a significant portion of the U.S. population, it is not representative of the entire country, as several states are not included in the database. In addition, SEER may not adequately capture areas with the highest levels of PFAS contamination in drinking water, which could limit the generalizability of our findings to communities most affected by PFAS. This limitation might partly explain the lack of observed associations in our study for cancers such as kidney and testicular cancers found in previous studies [36], as areas with the most severe contamination, such as Michigan [89], and potential health effects could be underrepresented. Future studies incorporating broader population-level data sources are necessary to further elucidate the relationship between PFAS exposure and cancer incidence.
In addition, we were unable to control for the lack of lagging between PFAS exposure and cancer incidence. It was particularly an issue for UCMR5 data since UCMR5 was conducted after cancer diagnosis. If we assume UCMR3 was a valid measure of PFAS exposure through water, there would be significant exposure misclassification when using UCMR5. Approximately half of the exposed water systems from UCMR3 would be misclassified as not exposed by UCMR5 while 11% of the unexposed water systems would be classified as exposed by UCMR5. However, the exposure classification would not be driven by cancer incidence and thus, it could result in bias in our effect estimates towards null.
Moreover, mitigation efforts may have been made towards water systems that have been previously detected with PFAS based on UCMR3 data and the mitigation effort may not have been made systematically resulting in further misclassification of PFAS exposure based on UCMR5.
In addition, we are unable to account for the various latency periods of cancers in our study. We are unable to assess whether PFAS monitoring data from both UCMR3 and UCMR5 was indeed a valid measure of historical levels of PFAS water contamination. Therefore, our results should be interpreted with caution.
We also did not account for multiple comparisons when conducting analyses of sub-organ systems because the cancer rate in major cancer systems may be driven primarily by one cancer (i.e. lung cancer for the respiratory system). Therefore, false positive associations were still likely.
In regions where PFAS contamination or water contamination is prevalent, people may not drink tap water. We did not have individual-level water drinking behavior data and therefore, nondifferential exposure misclassification of PFAS exposure from drinking water is possible and would bias our effect estimates towards null.
Conclusions
This cancer-wide ecological study presents evidence linking PFAS exposure through drinking water to increased cancer risks. The significant associations identified between PFAS in drinking water and various cancers, including those of the endocrine, digestive, oral cavity, pharynx, skin, and respiratory systems, underscore the urgent need for more comprehensive research. Given the recent regulation of PFAS in drinking water by the US EPA, our findings highlight the critical importance of developing effective strategies to mitigate cancer risks from exposure to PFAS through drinking water.
Data availability
Data were publicly available and downloaded from US EPA and National Cancer Institute websites. Codes were available upon request.
Code availability
Codes were available upon request.
References
Gaines LGT. Historical and current usage of per- and polyfluoroalkyl substances (PFAS): a literature review. Am J Ind Med. 2023;66:353–78.
Domingo JL, Nadal M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: a review of the recent scientific literature. Environ Res. 2019;177:108648.
Smalling KL, Romanok KM, Bradley PM, Morriss MC, Gray JL, Kanagy LK, et al. Per- and polyfluoroalkyl substances (PFAS) in United States tapwater: comparison of underserved private-well and public-supply exposures and associated health implications. Environ Int. 2023;178:108033.
US EPA. Final PFAS national primary drinking water regulation [Internet]. 2024 [cited 2024 Apr 16]. Available from: https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Boyd RI, Ahmad S, Singh R, Fazal Z, Prins GS, Madak Erdogan Z, et al. Toward a mechanistic understanding of poly- and perfluoroalkylated substances and cancer. Cancers. 2022;14:2919.
Soto AM, Sonnenschein C. Environmental causes of cancer: endocrine disruptors as carcinogens. Nat Rev Endocrinol. 2010;6:363–70.
Shirke AV, Radke EG, Lin C, Blain R, Vetter N, Lemeris C, et al. Expanded systematic evidence map for hundreds of per- and polyfluoroalkyl substances (PFAS) and comprehensive PFAS human health dashboard. Environ Health Perspect. 2024;132:026001.
Zahm S, Bonde JP, Chiu WA, Hoppin J, Kanno J, Abdallah M, et al. Carcinogenicity of perfluorooctanoic acid and perfluorooctanesulfonic acid. Lancet Oncol. 2024;25:16–7.
Seyyedsalehi MS, Boffetta P. Per- and poly-fluoroalkyl substances (PFAS) exposure and risk of kidney, liver, and testicular cancers: a systematic review and meta-analysis. La Medicina del Lavoro [Internet]. 2023 [cited 2024 May 13];114. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10627102/
Guidance on PFAS exposure, testing, and clinical follow-up [Internet]. Washington, D.C.: National Academies Press; 2022 [cited 2023 Dec 10]. Available from: https://www.nap.edu/catalog/26156
Zheng J, Liu S, Yang J, Zheng S, Sun B. Per- and polyfluoroalkyl substances (PFAS) and cancer: detection methodologies, epidemiological insights, potential carcinogenic mechanisms, and future perspectives. Sci Total Environ. 2024;953:176158.
Xu Y, Jurkovic-Mlakar S, Lindh CH, Scott K, Fletcher T, Jakobsson K, et al. Associations between serum concentrations of perfluoroalkyl substances and DNA methylation in women exposed through drinking water: a pilot study in Ronneby, Sweden. Environ Int. 2020;145:106148.
National Cancer Institute. Cancer trends progress report [Internet]. 2024 [cited 2024 Jun 21]. Available from: https://progressreport.cancer.gov
Li H, Hammarstrand S, Midberg B, Xu Y, Li Y, Olsson DS, et al. Cancer incidence in a Swedish cohort with high exposure to perfluoroalkyl substances in drinking water. Environ Res. 2022;204:112217.
Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant. Environ Health Perspect. 2013;121:1313–8.
Vieira VM, Hoffman K, Shin HM, Weinberg JM, Webster TF, Fletcher T. Perfluorooctanoic acid exposure and cancer outcomes in a contaminated community: a geographic analysis. Environ Health Perspect. 2013;121:318–23.
Alsen M, Leung AM, van Gerwen M. Per- and polyfluoroalkyl substances (PFAS) in community water systems (CWS) and the risk of thyroid cancer: an ecological study. Toxics. 2023;11:786.
US EPA O. Third unregulated contaminant monitoring rule [Internet]. 2015 [cited 2023 Oct 25]. Available from: https://www.epa.gov/dwucmr/third-unregulated-contaminant-monitoring-rule
US EPA. Fifth unregulated contaminant monitoring rule [Internet]. 2024 [cited 2023 Aug 19]. Available from: https://www.epa.gov/dwucmr/fifth-unregulated-contaminant-monitoring-rule
NCI. SEER. 2024 [cited 2024 May 13]. SEER incidence data. Available from: https://seer.cancer.gov/data/index.html
SimpleLab E. HydroShare. 2022 [cited 2024 May 22]. U.S. Community Water Systems Service Boundaries, v3.0.0. Available from: SimpleLab, EPIC (2022). U.S. Community Water Systems Service Boundaries, v3.0.0, HydroShare, http://www.hydroshare.org/resource/9ebc0a0b43b843b9835830ffffdd971e
Areej Arif MFM, Dua Mahmood Ch KN. Water pollution and industries. Pure Appl Biol. 2020;9:2214–24.
Borck R, Schrauth P. Population density and urban air quality. Reg Sci Urban Econ. 2021;86:103596.
Strosnider H. Rural and urban differences in air quality, 2008–2012, and community drinking water quality, 2010–2015 — United States. MMWR Surveill Summ [Internet]. 2017 [cited 2024 Oct 10];66. Available from: https://www.facebook.com/CDCMMWR
Thakrar SK, Balasubramanian S, Adams PJ, Azevedo IML, Muller NZ, Pandis SN, et al. Reducing mortality from air pollution in the united states by targeting specific emission sources. Environ Sci Technol Lett. 2020;7:639–45.
Hammer MS, van Donkelaar A, Li C, Lyapustin A, Sayer AM, Hsu NC, et al. Global estimates and long-term trends of fine particulate matter concentrations (1998–2018). Environ Sci Technol. 2020;54:7879–90.
van Donkelaar A, Martin RV, Li C, Burnett RT. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ Sci Technol. 2019;53:2595–611.
Ingram DD, Franco SJ. 2013 NCHS Urban-Rural Classification Scheme for Counties. Vital Health Stat 2. 2014;1–73.
CDC. Behavioral risk factor surveillance system [Internet]. 2024 [cited 2024 May 17]. Available from: https://www.cdc.gov/brfss/index.html
US EPA. PFAS analytic tools [Internet]. 2024. Available from: https://echo.epa.gov/trends/pfas-tools
Cserbik D, Casas M, Flores C, Paraian A, Haug LS, Rivas I, et al. Concentrations of per- and polyfluoroalkyl substances (PFAS) in paired tap water and blood samples during pregnancy. J Expo Sci Environ Epidemiol. 2024;34:90–6.
Hu XC, Tokranov AK, Liddie J, Zhang X, Grandjean P, Hart JE, et al. Tap water contributions to plasma concentrations of poly- and perfluoroalkyl substances (PFAS) in a Nationwide Prospective Cohort of U.S. Women. Environ Health Perspect. 2019;127:067006.
Chang CJ, Ish JL, Chang VC, Daniel M, Jones RR, White AJ. Exposure to per- and polyfluoroalkyl substances and breast cancer risk: a systematic review and meta-analysis of epidemiologic studies. Am J Epidemiol. 2024;193:1182–96.
Mastrantonio M, Bai E, Uccelli R, Cordiano V, Screpanti A, Crosignani P. Drinking water contamination from perfluoroalkyl substances (PFAS): an ecological mortality study in the Veneto Region, Italy. Eur J Public Health. 2018;28:180–5.
Steenland K, Winquist A. PFAS and cancer, a scoping review of the epidemiologic evidence. Environ Res. 2021;194:110690.
Moon J, Mun Y. The association between per- and polyfluoroalkyl substances (PFASs) and brain, esophageal, melanomatous skin, prostate, and lung cancer using the 2003–2018 US National Health and Nutrition Examination Survey (NHANES) datasets. Heliyon. 2024;10:e24337.
Costello E, Rock S, Stratakis N, Eckel SP, Walker DI, Valvi D, et al. Exposure to per- and polyfluoroalkyl substances and markers of liver injury: a systematic review and meta-analysis. Environ Health Perspect. 2022;130:046001.
Goodrich JA, Walker D, Lin X, Wang H, Lim T, McConnell R, et al. Exposure to perfluoroalkyl substances and risk of hepatocellular carcinoma in a multiethnic cohort. JHEP Rep. 2022;4:100550.
Weatherly LM, Shane HL, Lukomska E, Baur R, Anderson SE. Systemic toxicity induced by topical application of heptafluorobutyric acid (PFBA) in a murine model. Food Chem Toxicol. 2021;156:112528.
Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int. 2013;59:354–62.
Chen Z, Yang T, Walker DI, Thomas DC, Qiu C, Chatzi L, et al. Dysregulated lipid and fatty acid metabolism link perfluoroalkyl substances exposure and impaired glucose metabolism in young adults. Environ Int. 2020;145:106091.
Sen P, Qadri S, Luukkonen PK, Ragnarsdottir O, McGlinchey A, Jäntti S, et al. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J Hepatol. 2022;76:283–93.
Wu B, Pan Y, Li Z, Wang J, Ji S, Zhao F, et al. Serum per- and polyfluoroalkyl substances and abnormal lipid metabolism: a nationally representative cross-sectional study. Environ Int. 2023;172:107779.
Wu K, Lin F. Lipid metabolism as a potential target of liver cancer. J Hepatocell Carcinoma. 2024;11:327–46.
Laredo V, García-Mateo S, Martínez-Domínguez SJ, López de la Cruz J, Gargallo-Puyuelo CJ, Gomollón F. Risk of cancer in patients with inflammatory bowel diseases and keys for patient management. Cancers. 2023;15:871.
Gao B, Chen L, Xu W, Shan J, Shen W, Gao N. Effects of perfluorooctanoic acid on gut microbiota and microbial metabolites in C57BL/6J mice. Metabolites. 2023;13:707.
Rashid F, Dubinkina V, Ahmad S, Maslov S, Irudayaraj JMK. Gut microbiome-host metabolome homeostasis upon exposure to PFOS and GenX in male mice. Toxics. 2023;11:281.
Rashid F, Ahmad S, Irudayaraj JMK. Effect of perfluorooctanoic acid on the epigenetic and tight junction genes of the mouse intestine. Toxics. 2020;8:64.
Gieryńska M, Szulc-Dąbrowska L, Struzik J, Mielcarska MB, Gregorczyk-Zboroch KP. Integrity of the intestinal barrier: the involvement of epithelial cells and microbiota—a mutual relationship. Animals. 2022;12:145.
Li Q, von Ehrlich-Treuenstätt V, Schardey J, Wirth U, Zimmermann P, Andrassy J, et al. Gut barrier dysfunction and bacterial lipopolysaccharides in colorectal cancer. J Gastrointest Surg. 2023;27:1466–72.
Innes KE, Wimsatt JH, Frisbee S, Ducatman AM. Inverse association of colorectal cancer prevalence to serum levels of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in a large Appalachian population. BMC Cancer. 2014;14:45.
Huang SN, Hu YH, Xu TT, Luan YL, Zeng LX, Zhang ZF, et al. Exposure to per- and polyfluoroalkyl substances in lung cancer patients and their associations with clinical health indicators. Environ Pollut. 2024;350:123995.
Dragon J, Hoaglund M, Badireddy AR, Nielsen G, Schlezinger J, Shukla A. Perfluoroalkyl substances (PFAS) affect inflammation in lung cells and tissues. Int J Mol Sci. 2023;24:8539.
Alsen M, Sinclair C, Cooke P, Ziadkhanpour K, Genden E, van Gerwen M. Endocrine Disrupting Chemicals and Thyroid Cancer: An Overview. Toxics. 2021;9:14.
Coperchini F, Croce L, Ricci G, Magri F, Rotondi M, Imbriani M, et al. Thyroid disrupting effects of old and new generation PFAS. Front Endocrinol. 2021. https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2020.612320/full
Krashin E, Piekiełko-Witkowska A, Ellis M, Ashur-Fabian O. Thyroid hormones and cancer: a comprehensive review of preclinical and clinical studies. Front Endocrinol. 2019. https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2019.00059/full
Davidsen N, Ramhøj L, Lykkebo CA, Kugathas I, Poulsen R, Rosenmai AK, et al. PFOS-induced thyroid hormone system disrupted rats display organ-specific changes in their transcriptomes. Environ Pollut. 2022;305:119340.
Zhang S, Chen K, Li W, Chai Y, Zhu J, Chu B, et al. Varied thyroid disrupting effects of perfluorooctanoic acid (PFOA) and its novel alternatives hexafluoropropylene-oxide-dimer-acid (GenX) and ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA) in vitro. Environ Int. 2021;156:106745.
Li H, Yang M, Yang J, Seery S, Ma C, Liu Y, et al. Per- and polyfluoroalkyl substances and the associated thyroid cancer risk: a case-control study in China. Chemosphere. 2023;337:139411.
Madrigal JM, Troisi R, Surcel HM, Öhman H, Kivelä J, Kiviranta H, et al. Prediagnostic serum concentrations of per- and polyfluoroalkyl substances and risk of papillary thyroid cancer in the Finnish Maternity Cohort. Int J Cancer. 2024;154:979–91.
Liu M, Zhang G, Meng L, Han X, Li Y, Shi Y, et al. Associations between novel and legacy per- and polyfluoroalkyl substances in human serum and thyroid cancer: a case and healthy population in Shandong Province, East China. Environ Sci Technol. 2022;56:6144–51.
van Gerwen M, Colicino E, Guan H, Dolios G, Nadkarni GN, Vermeulen RCH, et al. Per- and polyfluoroalkyl substances (PFAS) exposure and thyroid cancer risk. eBioMedicine [Internet]. 2023 Nov 1 [cited 2024 May 28];97. Available from: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(23)00397-3/fulltext?keyword=Heel%20and%20arch%20pain
Xu FZ, Zheng LL, Chen KH, Wang R, Yi DD, Jiang CY, et al. Serum sex hormones correlate with pathological features of papillary thyroid cancer. Endocrine. 2024;84:148–54.
Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE. Perfluorinated chemicals as emerging environmental threats to kidney health: a scoping review. Clin J Am Soc Nephrol. 2018;13:1479.
Jones RR, Madrigal JM, Troisi R, Surcel HM, Öhman H, Kivelä J, et al. Maternal serum concentrations of per- and polyfluoroalkyl substances and childhood acute lymphoblastic leukemia. JNCI: J Natl Cancer Inst. 2024;116:728–36.
Brown-Leung JM, Cannon JR. Chapter Nine - Neurochemical mechanisms of perfluoroalkyl substances (PFAS) neurotoxic action. In: Kodavanti PRS, Aschner M, Costa LG, editors. Advances in neurotoxicology. Neurotoxicity of halogenated organic compounds, vol. 10 [Internet]. Academic Press; 2023. pp. 367–98.
Xie MY, Lin ZY, Liu LY, Wu CC, Liu YW, Huang GL, et al. Use of glioma to assess the distribution patterns of perfluoroalkyl and polyfluoroalkyl substances in human brain. Environ Res. 2022;204:112011.
Whitehead HD, Venier M, Wu Y, Eastman E, Urbanik S, Diamond ML, et al. Fluorinated compounds in North American cosmetics. Environ Sci Technol Lett. 2021;8:538–44.
Harris KJ, Munoz G, Woo V, Sauvé S, Rand AA. Targeted and suspect screening of per- and polyfluoroalkyl substances in cosmetics and personal care products. Environ Sci Technol. 2022;56:14594–604.
Bernardini I, Matozzo V, Valsecchi S, Peruzza L, Rovere GD, Polesello S, et al. The new PFAS C6O4 and its effects on marine invertebrates: first evidence of transcriptional and microbiota changes in the Manila clam Ruditapes philippinarum. Environ Int. 2021;152:106484.
Jaspers VLB, Herzke D, Eulaers I, Gillespie BW, Eens M. Perfluoroalkyl substances in soft tissues and tail feathers of Belgian barn owls (Tyto alba) using statistical methods for left-censored data to handle non-detects. Environ Int. 2013;52:9–16.
Koskela A, Koponen J, Lehenkari P, Viluksela M, Korkalainen M, Tuukkanen J. Perfluoroalkyl substances in human bone: concentrations in bones and effects on bone cell differentiation. Sci Rep. 2017;7:6841.
US EPA. Human health toxicity values for perfluorobutane sulfonic acid and related compound potassium perfluorobutane sulfonate [Internet]. 2021 [cited 2024 Jun 25]. Available from: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=350888
Messmer MF, Salloway J, Shara N, Locwin B, Harvey MW, Traviss N. Risk of cancer in a community exposed to per- and poly-fluoroalkyl substances. Environ Health Insights. 2022;16:11786302221076707.
National Cancer Institute. SEER. 2024 [cited 2024 May 28]. Cancer of the oral cavity and pharynx - Cancer Stat Facts. Available from: https://seer.cancer.gov/statfacts/html/oralcav.html
Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol. 2019;29:131–47.
Diaz OE, Sorini C, Morales RA, Luo X, Frede A, Krais AM, et al. Perfluorooctanesulfonic acid modulates barrier function and systemic T-cell homeostasis during intestinal inflammation. Dis Model Mech. 2021;14:dmm049104.
Chen Y, Paul KC, Walker DI, Jones DP, Wang X, Ritz BR, et al. Neonatal per- and polyfluoroalkyl substance exposure in relation to retinoblastoma. Environ Res. 2024;240:117435.
Roth K, Yang Z, Agarwal M, Liu W, Peng Z, Long Z, et al. Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environ Int. 2021;157:106843.
Wen ZJ, Wei YJ, Zhang YF, Zhang YF. A review of cardiovascular effects and underlying mechanisms of legacy and emerging per- and polyfluoroalkyl substances (PFAS). Arch Toxicol. 2023;97:1195–245.
Xie Z, Tan J, Fang G, Ji H, Miao M, Tian Y, et al. Associations between prenatal exposure to perfluoroalkyl substances and neurobehavioral development in early childhood: a prospective cohort study. Ecotoxicol Environ Saf. 2022;241:113818.
Cathey AL, Nguyen VK, Colacino JA, Woodruff TJ, Reynolds P, Aung MT. Exploratory profiles of phenols, parabens, and per- and poly-fluoroalkyl substances among NHANES study participants in association with previous cancer diagnoses. J Expo Sci Environ Epidemiol. 2023;33:687–98.
Winquist A, Hodge JM, Diver WR, Rodriguez JL, Troeschel AN, Daniel J, et al. Case–cohort study of the association between PFAS and selected cancers among participants in the American Cancer Society’s Cancer Prevention Study II LifeLink Cohort. Environ Health Perspect. 2023;131:127007.
Jain RB, Ducatman A. Serum concentrations of selected perfluoroalkyl substances for US females compared to males as they age. Sci Total Environ. 2022;842:156891.
Li Y, Andersson A, Xu Y, Pineda D, Nilsson CA, Lindh CH, et al. Determinants of serum half-lives for linear and branched perfluoroalkyl substances after long-term high exposure—a study in Ronneby, Sweden. Environ Int. 2022;163:107198.
Attanasio R. Sex differences in the association between perfluoroalkyl acids and liver function in US adolescents: analyses of NHANES 2013–2016. Environ Pollut. 2019;254:113061.
Palaniyandi J, Bruin JE, Kumarathasan P, MacPherson S, Borghese MM, Ashley-Martin J. Prenatal exposure to perfluoroalkyl substances and inflammatory biomarker concentrations. Environ Epidemiol. 2023;7:e262.
Michigan PFAS Action Response Team. PFAS sites and areas of interest [Internet]. 2024 [cited 2024 Oct 7]. Available from: https://www.michigan.gov/pfasresponse/investigations/sites-aoi
Acknowledgements
Support for this research was provided by a pilot grant from the Southern California Environmental Health Sciences Center (P30ES007048). Additional funding includes the University of Southern California Norris Comprehensive Cancer Center Support Grant (CCSG) (National Cancer Institute 5P30CA014089-47). LC was supported by NIH (U01HG013288, R01ES029944, R01ES030691, R01ES030364, and P30ES007048), and the European Union: The Advancing Tools for Human Early Lifecourse Exposome Research and Translation (ATHLETE) project, grant agreement number 874583.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium.
Author information
Authors and Affiliations
Contributions
SL contributed to conceptualization, methodology, formal analysis, investigation, data curation, writing-original draft, and funding acquisition. PO contributed to methodology, and writing-review and editing. LZ contributed to methodology and writing-review and editing. JAG contributed to methodology and writing-review and editing. RM contributed to methodology, writing-review and editing, and funding acquisition. DVC contributed to methodology, writing-review and editing, and funding acquisition. LC contributed to methodology, writing-review and editing, funding acquisition, and supervision. MA contributed to methodology, writing-review and editing, funding acquisition, and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following financial interests/personal relationships which may be considered potential competing interests: LC has served as an expert consultant for plaintiffs in litigation related to PFAS-contaminated drinking water. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate
All study protocols were reviewed and approved by the University of Southern California Institutional Review Board (UP-24-00198). Informed consent was waived (determined by the Institutional Review Board) per 45 CFR 46.102 (e)(1), as the project does not include information or biospecimens obtained through intervention or interaction with the individuals, and does not use, study, or analyze information or biospecimens or does not obtain, use, study, analyze, or generate identifiable private information or identifiable biospecimens.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, S., Oliva, P., Zhang, L. et al. Associations between per-and polyfluoroalkyl substances (PFAS) and county-level cancer incidence between 2016 and 2021 and incident cancer burden attributable to PFAS in drinking water in the United States. J Expo Sci Environ Epidemiol (2025). https://doi.org/10.1038/s41370-024-00742-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41370-024-00742-2