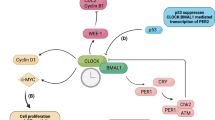
Abstract
Numerous physiological functions exhibit substantial circadian oscillations. In the kidneys, renal plasma flow, the glomerular filtration rate and tubular reabsorption and/or secretion processes have been shown to peak during the active phase and decline during the inactive phase. These functional rhythms are driven, at least in part, by a self-sustaining cellular mechanism termed the circadian clock. The circadian clock controls different cellular functions, including transcription, translation and protein post-translational modifications (such as phosphorylation, acetylation and ubiquitylation) and degradation. Disruption of the circadian clock in animal models results in the loss of blood pressure control and substantial changes in the circadian pattern of water and electrolyte excretion in the urine. Kidney-specific suppression of the circadian clock in animals implicates both the intrinsic renal and the extrarenal circadian clocks in these pathologies. Alterations in the circadian rhythm of renal functions are associated with the development of hypertension, chronic kidney disease, renal fibrosis and kidney stones. Furthermore, renal circadian clocks might interfere with the pharmacokinetics and/or pharmacodynamics of various drugs and are therefore an important consideration in the treatment of some renal diseases or disorders.
Key points
-
Several renal functions, including renal plasma flow, glomerular filtration rate, tubular transport activities and diuresis, have circadian rhythms.
-
Molecular clocks partially drive these oscillations and act on most intracellular processes (such as DNA replication, transcription, translation, post-translational modifications, protein sorting and membrane targeting).
-
Disruption of the molecular clock in mice leads to blood pressure abnormalities and impairs the circadian rhythmicity of water and sodium homeostasis.
-
The involvement of circadian rhythms and molecular clocks in human renal diseases remains uncertain, but chronopharmacology is emerging as a key player in blood pressure control.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rakova, N. et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell. Metabolism 17, 125–131 (2013).
Smith, E. On the elimination of urea and urinary water, in relation to the period of the day, season, exertion, food, prison discipline, weight of body and other influences acting in the cycle of the year. Phil. Trans. R. Soc. Lond. 151, 747–834 (1861).
Bonny, O. & Firsov, D. Circadian regulation of renal function and potential role in hypertension. Curr. Opin. Nephrol. Hypertens. 22, 439–444 (2013).
Firsov, D. & Bonny, O. Circadian regulation of renal function. Kidney Int. 78, 640–645 (2010).
Wuerzner, G., Firsov, D. & Bonny, O. Circadian glomerular function: from physiology to molecular and therapeutical aspects. Nephrol. Dial Transplant 29, 1475–1480 (2014).
Stow, L. R. & Gumz, M. L. The circadian clock in the kidney. J. Am. Soc. Nephrol. 22, 598–604 (2011).
Gumz, M. L. Molecular basis of circadian rhythmicity in renal physiology and pathophysiology. Exp. Physiol. 101, 1025–1029 (2016).
Solocinski, K. & Gumz, M. L. The circadian clock in the regulation of renal rhythms. J. Biol. Rhythms 30, 470–486 (2015).
Koopman, M. G. et al. Circadian rhythm of glomerular filtration rate in normal individuals. Clin. Sci. (Lond.) 77, 105–111 (1989).
Tokonami, N. et al. Local renal circadian clocks control fluid-electrolyte homeostasis and BP. J. Am. Soc. Nephrol. 25, 1430–1439 (2014).
Pons, M., Forpomes, O., Espagnet, S. & Cambar, J. Relationship between circadian changes in renal hemodynamics and circadian changes in urinary glycosaminoglycan excretion in normal rats. Chronobiol. Int. 13, 349–358 (1996).
Nikolaeva, S. et al. The circadian clock modulates renal sodium handling. J. Am. Soc. Nephrol. 23, 1019–1026 (2012).
Pons, M., Tranchot, J., L’Azou, B. & Cambar, J. Circadian rhythms of renal hemodynamics in unanesthetized, unrestrained rats. Chronobiol. Int. 11, 301–308 (1994).
Steele, A. et al. What is responsible for the diurnal variation in potassium excretion? Am. J. Physiol. 267, R554–560 (1994).
Fujii, T. et al. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am. J. Kidney Dis. 33, 29–35 (1999).
Hara, M. et al. Robust circadian clock oscillation and osmotic rhythms in inner medulla reflecting cortico-medullary osmotic gradient rhythm in rodent kidney. Sci. Rep. 7, 7306 (2017).
Emans, T. W., Janssen, B. J., Joles, J. A. & Krediet, C. T. P. Circadian rhythm in kidney tissue oxygenation in the rat. Front. Physiol. 8, 205 (2017).
Voogel, A. J., Koopman, M. G., Hart, A. A., van Montfrans, G. A. & Arisz, L. Circadian rhythms in systemic hemodynamics and renal function in healthy subjects and patients with nephrotic syndrome. Kidney Int. 59, 1873–1880 (2001).
Somers, V. K., Dyken, M. E., Mark, A. L. & Abboud, F. M. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 328, 303–307 (1993).
Moore-Ede, M. C. Physiology of the circadian timing system: predictive versus reactive homeostasis. Am. J. Physiol. 250, R737–R752 (1986).
Callaway, E. & Ledford, H. Medicine Nobel awarded for work on circadian clocks. Nature 550, 18 (2017).
Dibner, C., Schibler, U. & Albrecht, U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549 (2010).
Atger, F., Mauvoisin, D., Weger, B., Gobet, C. & Gachon, F. Regulation of mammalian physiology by interconnected circadian and feeding rhythms. Front. Endocrinol. 8, 42 (2017).
Meszaros, K. et al. Development of the circadian clockwork in the kidney. Kidney Int. 86, 915–922 (2014).
Mazzoccoli, G. et al. Clock gene expression in mouse kidney and testis: analysis of periodical and dynamical patterns. J. Biol. Regulators Homeostat. Agents 26, 303–311 (2012).
Wu, T. et al. Regulation of circadian gene expression in the kidney by light and food cues in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R635–641 (2010).
Evans, J. A. Collective timekeeping among cells of the master circadian clock. J. Endocrinol. 230, R27–R49 (2016).
Fernandez, D. C., Chang, Y. T., Hattar, S. & Chen, S. K. Architecture of retinal projections to the central circadian pacemaker. Proc. Natl Acad. Sci. USA 113, 6047–6052 (2016).
Schibler, U. et al. Clock-talk: interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb. Symp. Quant. Biol. 80, 223–232 (2015).
Luck, S. & Westermark, P. O. Circadian mRNA expression: insights from modeling and transcriptomics. Cell. Mol. Life Sci. 73, 497–521 (2016).
Zhang, R., Lahens, N. F., Ballance, H. I., Hughes, M. E. & Hogenesch, J. B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl Acad. Sci. USA 111, 16219–16224 (2014).
Panda, S. et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 (2002).
Storch, K. F. et al. Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83 (2002).
Zuber, A. M. et al. Molecular clock is involved in predictive circadian adjustment of renal function. Proc. Natl Acad. Sci. USA 106, 16523–16528 (2009).
Pradervand, S., Zuber Mercier, A., Centeno, G., Bonny, O. & Firsov, D. A comprehensive analysis of gene expression profiles in distal parts of the mouse renal tubule. Pflugers Arch. 460, 925–952 (2010).
Salhi, A., Centeno, G., Firsov, D. & Crambert, G. Circadian expression of H,K-ATPase type 2 contributes to the stability of plasma K(+) levels. FASEB J. 26, 2859–2867 (2012).
Pouly, D. et al. USP2-45 is a circadian clock output effector regulating calcium absorption at the post-translational level. PLOS One 11, e0145155 (2016).
Jouffe, C. et al. The circadian clock coordinates ribosome biogenesis. PLOS Biol. 11, e1001455 (2013).
Atger, F. et al. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proc. Natl Acad. Sci. USA 112, E6579–6588 (2015).
Castelo-Szekely, V., Arpat, A. B., Janich, P. & Gatfield, D. Translational contributions to tissue specificity in rhythmic and constitutive gene expression. Genome Biol 18, 116 (2017).
Susa, K. et al. WNK-OSR1/SPAK-NCC signal cascade has circadian rhythm dependent on aldosterone. Biochem. Biophys. Res. Commun. 427, 743–747 (2012).
Ivy, J. R. et al. Glucocorticoids induce nondipping blood pressure by activating the thiazide-sensitive cotransporter. Hypertension 67, 1029–1037 (2016).
Hirano, A., Fu, Y. H. & Ptacek, L. J. The intricate dance of post-translational modifications in the rhythm of life. Nature Struct. Mol. Biol. 23, 1053–1060 (2016).
Robles, M. S., Humphrey, S. J. & Mann, M. Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell. Metab. 25, 118–127 (2017).
Mauvoisin, D. et al. Circadian and feeding rhythms orchestrate the diurnal liver acetylome. Cell Rep. 20, 1729–1743 (2017).
Rossier, B. C., Baker, M. E. & Studer, R. A. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol. Rev. 95, 297–340 (2015).
Hurwitz, S., Cohen, R. J. & Williams, G. H. Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged bed rest. J. Appl. Physiol. 96, 1406–1414 (2004).
Doi, M. et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat. Med. 16, 67–74 (2009).
Adamovich, Y., Ladeuix, B., Golik, M., Koeners, M. P. & Asher, G. Rhythmic oxygen levels reset circadian clocks through HIF1alpha. Cell. Metabolism 25, 93–101 (2017).
Walton, Z. E. et al. Acid suspends the circadian clock in hypoxia through inhibition of mTOR. Cell 174, 72–87 (2018).
Richards, J. et al. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am. J. Physiol. Renal Physiol. 305, F1697–1704 (2013).
Solocinski, K. et al. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiol. 220, 72–82 (2017).
Douma, L. G. et al. Renal Na handling defect associated with PER1-dependent non-dipping hypertension in male mice. Am. J. Physiol. Renal Physiol. 314, F1138–F1144 (2018).
Gumz, M. L. et al. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J. Clin. Invest. 119, 2423–2434 (2009).
Gumz, M. L. et al. Regulation of alphaENaC expression by the circadian clock protein Period 1 in mpkCCD(c14) cells. Biochim. Biophys. Acta 1799, 622–629 (2010).
Richards, J., Jeffers, L. A., All, S. C., Cheng, K. Y. & Gumz, M. L. Role of Per1 and the mineralocorticoid receptor in the coordinate regulation of alphaENaC in renal cortical collecting duct cells. Front.Physiol. 4, 253 (2013).
Richards, J. et al. A role for the circadian clock protein Per1 in the regulation of the NaCl co-transporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J. Biol. Chem. 289, 11791–11806 (2014).
Solocinski, K. et al. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am. J. Physiol. Renal Physiol. 309, F933–942 (2015).
Richards, J. et al. Tissue-specific and time-dependent regulation of the endothelin axis by the circadian clock protein Per1. Life Sci. 118, 255–262 (2014).
Ge, Y. et al. Endogenously produced 20-HETE modulates myogenic and TGF response in microperfused afferent arterioles. Prostaglandins Other Lipid Mediat. 102–103, 42–48 (2013).
Yu, M., Lopez, B., Dos Santos, E. A., Falck, J. R. & Roman, R. J. Effects of 20-HETE on Na+ transport and Na+ -K+ -ATPase activity in the thick ascending loop of Henle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R2400–R2405 (2007).
Quigley, R., Baum, M., Reddy, K. M., Griener, J. C. & Falck, J. R. Effects of 20-HETE and 19(S)-HETE on rabbit proximal straight tubule volume transport. Am. J. Physiol. Renal Physiol. 278, F949–953 (2000).
Chen, W. D. et al. Circadian CLOCK mediates activation of transforming growth factor-beta signaling and renal fibrosis through cyclooxygenase 2. Am. J. Pathol. 185, 3152–3163 (2015).
Bunger, M. K. et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 (2000).
Rudic, R. D. et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLOS Biol. 2, e377 (2004).
Shimba, S. et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLOS One 6, e25231 (2011).
Kondratov, R. V., Kondratova, A. A., Gorbacheva, V. Y., Vykhovanets, O. V. & Antoch, M. P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 20, 1868–1873 (2006).
Bunger, M. K. et al. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis 41, 122–132 (2005).
Boden, M. J., Varcoe, T. J., Voultsios, A. & Kennaway, D. J. Reproductive biology of female Bmal1 null mice. Reprod. (Cambridge, Engl.) 139, 1077–1090 (2010).
Curtis, A. M. et al. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc. Natl Acad. Sci. USA 104, 3450–3455 (2007).
Xie, Z. et al. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Invest. 125, 324–336 (2015).
Sequeira Lopez, M. L., Pentz, E. S., Nomasa, T., Smithies, O. & Gomez, R. A. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev. Cell 6, 719–728 (2004).
Storch, K. F. et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell 130, 730–741 (2007).
Nikolaeva, S. et al. Nephron-specific deletion of circadian clock gene Bmal1 alters the plasma and renal metabolome and impairs drug disposition. J. Am. Soc. Nephrol. 27, 2997–3004 (2016).
Traykova-Brauch, M. et al. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat. Med. 14, 979–984 (2008).
Vallon, V. et al. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am. J. Physiol. Renal Physiol. 302, F1293–F1299 (2012).
Toh, K. L. et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291, 1040–1043 (2001).
Hirano, A. et al. A Cryptochrome 2 mutation yields advanced sleep phase in humans. eLife 5, e16695 (2016).
Xu, Y. et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 434, 640–644 (2005).
Patke, A. et al. Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder. Cell 169, 203–215 (2017).
Jagannath, A., Taylor, L., Wakaf, Z., Vasudevan, S. R. & Foster, R. G. The genetics of circadian rhythms, sleep and health. Human Mol. Genet. 26, R128–R138 (2017).
Dashti, H. S. et al. Clock Genes Explain a Large Proportion of Phenotypic Variance in Systolic Blood Pressure and This Control Is Not Modified by Environmental Temperature. Am. J. Hypertension 29, 132–140 (2016).
Woon, P. Y. et al. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc. Natl Acad. Sci. USA 104, 14412–14417 (2007).
Scheer, F. A., Hilton, M. F., Mantzoros, C. S. & Shea, S. A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl Acad. Sci. USA 106, 4453–4458 (2009).
Martino, T. A. et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1675–1683 (2008).
Fletcher, E. K. et al. Deoxycorticosterone/salt-mediated cardiac inflammation and fibrosis are dependent on functional CLOCK signaling in male mice. Endocrinology 158, 2906–2917 (2017).
Speed, J. S. et al. Diurnal pattern in skin Na(+) and water content is associated with salt-sensitive hypertension in ETB receptor deficient rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 314, R544–R551 (2017).
Yuan, D. et al. Blue light reduces organ injury from ischemia and reperfusion. Proc. Natl Acad. Sci. USA 113, 5239–5244 (2016).
Katayama, T. et al. Long-term renal denervation normalizes disrupted blood pressure circadian rhythm and ameliorates cardiovascular injury in a rat model of metabolic syndrome. J. Am. Heart Assoc. 2, e000197 (2013).
Singh, R. K. & Bansal, A. Studies on circadian periodicity of serum and urinary urate in healthy Indians and renal stone formers. Prog. Clin. Biol. Res. 227B, 305–313 (1987).
Singh, R. K., Bansal, A., Bansal, S. K. & Rai, S. P. Circadian variations of blood and urinary constituents in renal stone formers. Prog. Clin. Biol. Res. 341B, 551–557 (1990).
Singh, R. K., Bansal, A., Bansal, S. K., Singh, A. K. & Mahdi, A. A. Circadian periodicity of urinary inhibitor of calcium oxalate crystallization in healthy Indians and renal stone formers. Eur. Urol. 24, 387–392 (1993).
Sidhu, H. et al. The loss of circadian rhythmicity of urinary solute excretion in idiopathic stone formers. Br. J. Urol. 64, 333–335 (1989).
Touitou, Y. et al. Alterations in circadian rhythmicity in calcium oxalate renal stone formers. Int. J. Chronobiol. 8, 175–192 (1983).
Robert, M. et al. Circadian variations in the risk of urinary calcium oxalate stone formation. Br. J. Urol. 74, 294–297 (1994).
Bilobrov, V. M., Chugaj, A. V. & Bessarabov, V. I. Urine pH variation dynamics in healthy individuals and stone formers. Urol. Intern. 45, 326–331 (1990).
Cameron, M. et al. The diurnal variation in urine acidification differs between normal individuals and uric acid stone formers. Kidney Int. 81, 1123–1130 (2012).
Koopman, M. G. & Arisz, L. Spectrum of diurnal rhythms in glomerular permeability in patients with membranous nephropathy. Nephrol. Dial. Transplant. 12 (Suppl. 2), 47–52 (1997).
Koopman, M. G., Krediet, R. T., Koomen, G. C., Strackee, J. & Arisz, L. Circadian rhythm of proteinuria: consequences of the use of urinary protein:creatinine ratios. Nephrol. Dial. Transplant. 4, 9–14 (1989).
Lin, L. et al. Nocturnal and circadian rhythm of blood pressure is associated with renal structure damage and function in patients with IgAN. Arch. Med. Res. 47, 25–32 (2016).
Szelestei, T., Kovacs, T., Barta, J. & Nagy, J. Circadian blood pressure changes and cardiac abnormalities in IgA nephropathy. Am. J. Nephrol. 19, 546–551 (1999).
Skrzypczyk, P., Mizerska-Wasiak, M., Jerszow, B., Ruszczykowski, P. & Panczyk-Tomaszewska, M. Ambulatory arterial stiffness index, blood pressure variability, and nocturnal blood pressure dip in children with IgA and Henoch-Schonlein nephropathy. Clin. Nephrol. 87, 301–309 (2017).
Haruhara, K. et al. Circadian blood pressure abnormalities in patients with primary nephrotic syndrome. Clin. Exp. Hypertension 39, 155–159 (2017).
Uzu, T. et al. Thiazide diuretics enhance nocturnal blood pressure fall and reduce proteinuria in immunoglobulin A nephropathy treated with angiotensin II modulators. J. Hypertension 23, 861–865 (2005).
Velasquez, M. T., Beddhu, S., Nobakht, E., Rahman, M. & Raj, D. S. Ambulatory blood pressure in chronic kidney disease: ready for prime time? Kidney Int. Rep. 1, 94–104 (2016).
Russcher, M. et al. An observational study on disturbed peripheral circadian rhythms in hemodialysis patients. Chronobiol. Int. 32, 848–857 (2015).
Koch, B. C., Nagtegaal, J. E., Hagen, E. C., Wee, P. M. & Kerkhof, G. A. Different melatonin rhythms and sleep-wake rhythms in patients on peritoneal dialysis, daytime hemodialysis and nocturnal hemodialysis. Sleep Med. 11, 242–246 (2010).
Koch, B. C. et al. Effects of nocturnal hemodialysis on melatonin rhythm and sleep-wake behavior: an uncontrolled trial. Am. J. Kidney Dis. 53, 658–664 (2009).
Russcher, M. et al. The effects of kidney transplantation on sleep, melatonin, circadian rhythm and quality of life in kidney transplant recipients and living donors. Nephron 129, 6–15 (2015).
Aizman, R. I., Rabinowitz, L. & Mayer-Harnisch, C. Circadian rhythms and time course of adaptive sodium and potassium excretion in rats after uninephrectomy. Am. J. Physiol. 266, R1454–R1462 (1994).
Ohashi, N. et al. The effects of unilateral nephrectomy on blood pressure and its circadian rhythm. Internal Med. 55, 3427–3433 (2016).
Suzuki, J. et al. A critical role of sympathetic nerve regulation for the treatment of impaired daily rhythm in hypertensive Dahl rats. Hypertens. Res. 33, 1060–1065 (2010).
Ott, C. et al. Impact of renal denervation on tissue Na(+) content in treatment-resistant hypertension. Clin. Res. Cardiol. 107, 42–48 (2018).
Mochel, J. P. et al. Influence of feeding schedules on the chronobiology of renin activity, urinary electrolytes and blood pressure in dogs. Chronobiol. Int. 31, 715–730 (2014).
Hermida, R. C., Ayala, D. E., Fernandez, J. R. & Calvo, C. Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension 51, 69–76 (2008).
Farah, R., Makhoul, N., Arraf, Z. & Khamisy-Farah, R. Switching therapy to bedtime for uncontrolled hypertension with a nondipping pattern: a prospective randomized-controlled study. Blood Pressure Monitor. 18, 227–231 (2013).
Wang, C. et al. Effect of valsartan with bedtime dosing on chronic kidney disease patients with nondipping blood pressure pattern. J. Clin. Hypertension 15, 48–54 (2013).
Hermida, R. C., Ayala, D. E., Mojon, A. & Fernandez, J. R. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J. Am. Soc. Nephrol. 22, 2313–2321 (2011).
Crespo, J. J. et al. Administration-time-dependent effects of hypertension treatment on ambulatory blood pressure in patients with chronic kidney disease. Chronobiology Int. 30, 159–175 (2013).
Zhao, P., Xu, P., Wan, C. & Wang, Z. Evening versus morning dosing regimen drug therapy for hypertension. Cochrane Database Syst. Rev. 10, CD004184 (2011).
Liu, X. et al. Evening -versus morning- dosing drug therapy for chronic kidney disease patients with hypertension: a systematic review. Kidney Blood Pressure Res. 39, 427–440 (2014).
Wang, C. et al. Chronotherapy for hypertension in patients with chronic kidney disease: a systematic review and meta-analysis in non-black patients. Int. Urol. Nephrol. 49, 651–659 (2017).
Wang, C. et al. Evening versus morning dosing regimen drug therapy for chronic kidney disease patients with hypertension in blood pressure patterns: a systematic review and meta-analysis. Internal Med. J. 47, 900–906 (2017).
Castagna, A. et al. Circadian exosomal expression of renal thiazide-sensitive NaCl cotransporter (NCC) and prostasin in healthy individuals. Proteomics. Clin. Appl. 9, 623–629 (2015).
Uzu, T. et al. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation 96, 1859–1862 (1997).
Dhaun, N. et al. Diurnal variation in blood pressure and arterial stiffness in chronic kidney disease: the role of endothelin-1. Hypertension 64, 296–304 (2014).
Johnston, J. G., Speed, J. S., Jin, C. & Pollock, D. M. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am. J. Physiol. Renal Physiol. 311, F991–F998 (2016).
Thurley, K. et al. Principles for circadian orchestration of metabolic pathways. Proc. Natl Acad. Sci. USA 114, 1572–1577 (2017).
Reddy, P. et al. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell 38, 701–710 (1984).
Bargiello, T. A. & Young, M. W. Molecular genetics of a biological clock in Drosophila. Proc. Natl Acad. Sci. USA 81, 2142–2146 (1984).
Koch, B. C. et al. The effects of melatonin on sleep-wake rhythm of daytime haemodialysis patients: a randomized, placebo-controlled, cross-over study (EMSCAP study). Br. J. Clin. Pharmacol. 67, 68–75 (2009).
Acknowledgements
The authors thank their students, postdoctoral fellows and technicians, who contributed substantially to the advancement of the field through their curiosity and hard work in the laboratory. The authors are supported by grants from the Swiss National Science Foundation (31003A-169493 to D.F. and 310030–163340 to O.B.).
Author information
Authors and Affiliations
Contributions
Both authors contributed to all aspects of the conception, content, revisions and editing of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
NHGRI-EBI GWAS Catalogue: http://www.ebi.ac.uk/gwas/
Rights and permissions
About this article
Cite this article
Firsov, D., Bonny, O. Circadian rhythms and the kidney. Nat Rev Nephrol 14, 626–635 (2018). https://doi.org/10.1038/s41581-018-0048-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-018-0048-9
This article is cited by
-
Association between physical frailty, circadian syndrome and cardiovascular disease among middle-aged and older adults: a longitudinal study
BMC Geriatrics (2024)
-
Genetic correlation and mendelian randomization reveal the impact of sleep traits on urolithiasis risk
Scientific Reports (2024)
-
Disruption of circadian rhythm as a potential pathogenesis of nocturia
Nature Reviews Urology (2024)
-
Urine albumin-to-creatinine ratio diurnal variation rate predicts outcomes in idiopathic membranous nephropathy
Clinical and Experimental Nephrology (2024)
-
Integrated multi-omics and bioinformatic methods to reveal the mechanisms of sinomenine against diabetic nephropathy
BMC Complementary Medicine and Therapies (2023)